Potent Estrogen Receptor Modulator Bearing Carborane (Chem. & Biol. 2001)
Medicinal Chemistry
Application
of New Hydrophobic Structures for Drug Design
Carboranes (dicarba-closo-dodecaboranes) are a class of carbon-containing polyhedral boron-cluster compounds having remarkable thermal stability and exceptional hydrophobic character. The carboranes were utilized in medicinal chemistry in the field of boron neutron capture therapy (BNCT) for incorporation of large numbers of boron atoms into tumor cells. However, little attention has been paid to the possible use of carborane as a hydrophobic skeletal structure, in the field of drug design. We have recognized the importance of hydrophobic interaction in receptor-ligand complexation. The difference of binding constants between a ligand having a suitable hydrophobic group and a ligand without such a group sometimes reaches 100~10000 times. Therefore, we focused on the design and synthesis of nuclear receptor modulators (retinoid agonists, retinoid antagonists, estrogen agonists estrogen antagonists) bearing a carborane cage as a hydrophobic skeletal structure for evaluation of the utility of icosahedral carborane as a hydrophobic component for drug design.
For example, we designed candidate estrogen-receptor-binding compounds having carborane as a hydrophobic moiety and synthesized them. The most potent compound bearing a carborane cage (BE120) exhibited potent activity in the concentration range of 1x10-10 - 1x10-8 M by luciferase reporter gene assay; its potency is at least 10-fold greater than that of estradiol. The compound also showed potent in vivo effects on the recovery of uterine weight and bone loss in OVX mice. Development of the potent carborane-containing estrogenic agonists described here should yield novel candidate therapeutic agents, especially selective estrogen receptor modulators. Furthermore, the suitability of the spherical carborane cage for binding to the cavity of ER-LBD should provide a basis for a similar approach to developing novel ligands for other steroid receptors.

Potent Estrogen Receptor Modulator Bearing Carborane (Chem. & Biol. 2001)
Design and Synthesis of Modulators for Signal Transduction in Cells
Tumor-promoter, Teleocidins and their active congeners (indolactams) are
known to exist in an equilibrium between at least two conformational states
in solution, the twist and sofa form, due to cis-trans isomerization of
the amide bond and the steric effects of substituents on the nine-membered
lactam ring. Benzolactam-Vs, in which the indole ring of indolactams
is replaced with a benzene ring, were designed and synthesized in an attempt
to reproduce the active conformation of teleocidins. Among these benzolactams,
8-membered lactams (benzolactam-V8) can only exist in the twist form and
9- and 10-membered lactams (benzolactam-V9 and V10) exist exclusively in
the sofa form in solution. The stronger biological activity
of benzolactam-V-8-310 than that of indolactam-V (IL-V) and the inactivity
of benzolactam-V-9-310 for differentiation inducing activity of HL-60 clearly
indicated that the twist form is close to the active conformation of teleocidins.

The Two Stable Conformations of Teleocidin, and Synthetic Compounds Reproducing
the Active Conformation
(J. Am. Chem. Soc. 1996, J. Med. Chem. 1998)
Design and Synthesis of p-Glycoprotein Inhibitors for Reversing Taxol-Resistance
in Cancer Cell Lines
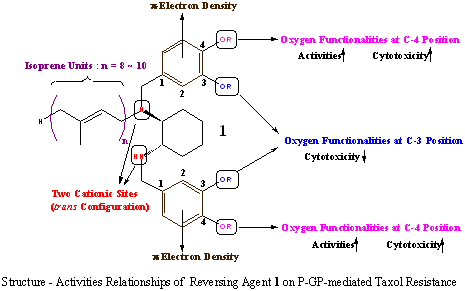
Several new types of chemotherapeutic agents to cancers, such as taxol, CPT-11 and erythropoetin, have become available on the basis of gene analysis during the last decade. However, multi drug resistance (MDR) to those agents has become a major obstacle to effective chemotherapy. One of mechanisms of MDR is overexpression of P-glycoprotein (P-GP), which is coded by mdr1 gene. P-GP is believed to function as an energy-dependent efflux pump of several chemotherapeutic agents, such as taxol and doxorubicin. Since P-GP inhibitors will become effective reversing agents for MDR, design and synthesis of new P-GP inhibitors have been one of central interests in the area of cancer chemotherapy.
Recently, we have investigated that some compounds with isoprene units (isoprenoids) completely reversed MDR in cultured MDR cell-lines. And we have screened many synthetic isoprenoids and have found isoprenoid 1 which reversed completely P-GP-mediated taxol resistance in KK47/TX, which was established from a human KK47 bladder cancer cell line. We have just begun to investigate more effective P-GP inhibitors on the basis of structure-activities relationships of compound 1.
Search for Natural Biologically Active Compounds with a New Skeletal Structure
Abstract