publication (2001 - 2005)
20051,2-Dicarba-closo-dodecaboran-1-yl naphthalene derivatives.
Kiminori Ohta, Tokuhito Goto, Yasuyuki Endo
Inorg. Chem. 2005, 44, 8569–8573.
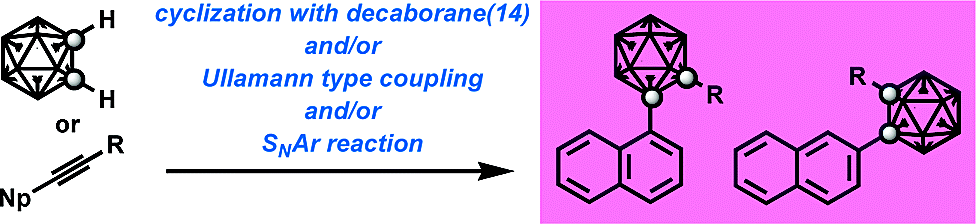
Design and synthesis of novel androgen receptor (AR) antagonists with sterically bulky icosahedral carboranes.
Tokuhito Goto, Kiminori Ohta, Tomoharu Suzuki, Shigeru Ohta, Yasuyuki Endo
Bioorg. Med. Chem. 2005, 13, 6414–6424.
A comparison of mesogenic properties of p-carborane-1,12-dicarbaldehyde schiff bases with their terephthaldehyde analogues.
Takashi Nagamine, Adam Januszko, Kiminori Ohta, Piotr Kaszynski, Yasuyuki Endo
Liq. Cryst. 2005, 32, 985–995.
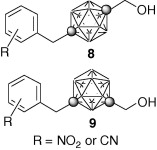
Potent androgen antagonists based on carborane as a hydrophobic core structure.
Shinya Fujii, Tokuhito Goto, Kiminori Ohta, Yuichi Hashimoto, Tomoharu Suzuki, Shigeru Ohta, Yasuyuki Endo
J. Med. Chem. 2005, 48, 4654–4662.
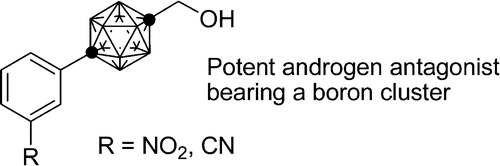
Synthesis of distorted molecules based on spatial control with icosahedral carboranes.
Yasuyuki Endo, Chalermkiat Songkram, Kiminori Ohta, Kentaro Yamaguchi
J. Organomet. Chem. 2005, 690, 2750–2756.
Potent estrogen receptor ligands based on bisphenols with a globular hydrophobic core.
Yasuyuki Endo, Tomohiro Yoshimi, Kiminori Ohta, Tomoharu Suzuki, Shigeru Ohta
J. Med. Chem. 2005, 48, 3941–3944.
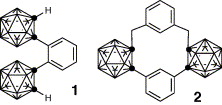
Regioselective synthesis of triiodo-o-carboranes and tetraiodo-o-carborane.
Hiroto Yamazaki, Kiminori Ohta, Yasuyuki Endo
Tetrahedron Lett. 2005, 46, 3119–3122.
Distorted benzene bearing two bulky substituents on adjacent positions: Structure of 1,2-bis- (1,2-dicarba-closo-dodecaboran-1-yl)benzene.
Yasuyuki Endo, Chalermkiat Songkram, Kiminori Ohta, Piotr Kaszynski, Kentaro Yamaguchi
Tetrahedron Lett. 2005, 46, 699–702.
New synthetic method of 1,2-diaryl-1,2-dicarba-closo-dodecaboranes employing aromatic nucleophilic substitution (SNAr) reaction.
Kiminori Ohta, Tokuhito Goto, Yasuyuki Endo
Tetrahedron Lett. 2005, 46, 483–485.
2004
Novel retinoid X receptor (RXR) antagonists having a dicarba-closo-dodecaborane as a hydrophobic moiety.
Kiminori Ohta, Toru Iijima, Emiko Kawachi, Hiroyuki Kagechika, Yasuyuki Endo
Bioorg. Med. Chem. Lett. 2004, 14, 5913–5918.
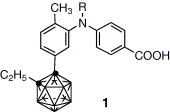
A new application of inorganic cluster, carboranes for medicinal drug design and molecular construction.
Yasuyuki Endo, Kiminori Ohta, Tomohiro Yoshimi, Kentaro Yamaguchi
Phosphorus, Sulfur and Silicon and the Related Elements 2004, 179, 799–802.
Structural effects in three-ring mesogenic derivatives of p-carborane and their hydrocarbon analogues.
Kiminori Ohta, Adam Januszko, Piotr Kaszynski, Takashi Nagamine, Genadz Sasnouski, Yasuyuki Endo
Liq. Cryst. 2004, 31, 671–682.
2002
Novel retinoid X receptor antagonists: Specific inhibition of retinoid synergism in RXR-RAR heterodimer actions.
Bitoku Takahashi, Kiminori Ohta, Emiko Kawachi, Hiroshi Fukusawa, Yuichi Hashimoto, Hiroyuki Kagechika
J. Med. Chem. 2002, 45, 3327–3330.
2001
Novel retinoidal tropolone derivatives. Bioisosteric relationship of tropolone ring with benzoic acid moiety in retinoid structure.
Masayuki Ebisawa, Kiminori Ohta, Emiko Kawachi, Hiroshi Fukusawa, Yuichi Hashimoto, Hiroyuki Kagechika
Chem. Pharm. Bull. 2001, 49, 501–503.

